The essence of dishwashing is hidden from the eyes.
Serving in clean dishes is one of the most self-evident things in hospitality. However, behind well-cleaned dishes lies the careful selection of appropriate cleaning agents. The essence of properly cleaned dishes is, in fact, hidden from the eyes, but it is important for the health and satisfaction of guests.
pH scale, which ranges from a pH value of 0 to a pH value of 14, is divided into two parts, where acidic substances are located between pH values of 0 and pH values of 7, while basic substances are found between pH values of 7 and pH values of 14. Neutral substances, such as distilled water, have a pH value of around 7. These values play an important role in selecting cleaning agents depending on the type of dirt we are dealing with..jpg)
To remove fats, basic substances are used, as basic environments allow for the dissolution of fat molecules. The more basic the substance, the better and faster it will remove fat. To remove deposits, such as limescale or minerals in water, acidic substances are used. Acids break down calcium and magnesium salts that form deposits and allow for their effective removal. The more acidic the substance, the faster it will remove deposits.
By logical reasoning, we might conclude that for the best cleaned dishes, we should use the most basic cleaning agent. However, there is another important factor we need to consider when cleaning products that come into contact with skin, such as plates, glasses, cutlery, and clothing. This is the pH of the skin, which in most people ranges between a pH value of 5.5 and a pH value of 5.7. The skin has a natural protective barrier whose acidity is important for maintaining health. If products that come into contact with skin have a pH value after cleaning that differs from the pH of the skin, it can cause irritation and rashes. It is important to carefully choose cleaning agents, as an incorrect pH can damage the skin's natural protective layer, increasing the risk of infections.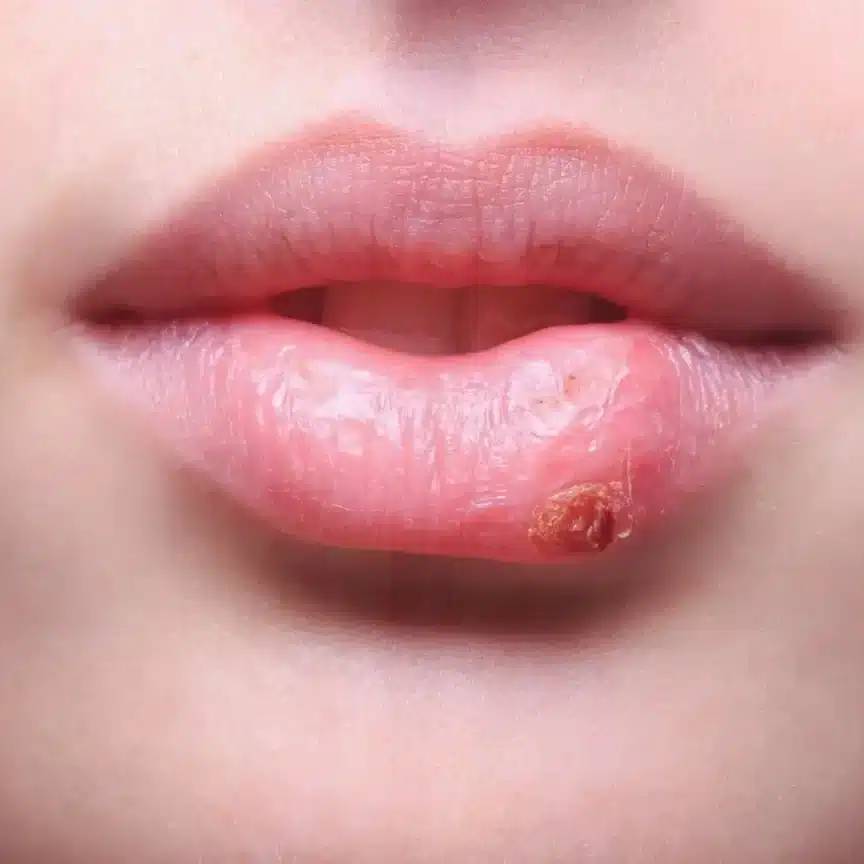
Therefore, after cleaning with a base, the use of acidic agents is also necessary. The most well-known are, for example, brilliant (for dishes) and fabric softener (for clothing). Their main purpose is not to create a pleasant fragrance or shine, but to lower the pH value of the cleaned products to a level as close as possible to the pH of the skin. This process is especially important to prevent long-term consequences, such as dry skin or damage caused by an overly alkaline environment. If we need to raise the pH value of a cleaned item to remove fats, we must then lower it to the proper level before finishing the wash. When cleaning clothing, we aim for it to have a pH value of 5 when wet and a pH value of 5.5 when dry. The same applies to dishes, which must have a pH value of 5.5 after washing. If we fail to lower the pH value to the proper level, skin irritation, especially on the mucous membranes (e.g., lips), that comes into contact with such dishes, may occur, leading to inflammation similar to herpes or other rashes. Regular pH value checks when cleaning, either using pH strips or more precise testing methods, are essential for ensuring proper hygiene and user safety.

In our stores, you can find high-quality and reputable cleaning agents from the brands Sanitec and Matrix, from the Italian manufacturer Italchimica. These cleaning agents have been developed with user safety and environmental impact in mind, as they are gentle on the skin and the environment. By using cleaning agents with the appropriate pH value, we ensure that products that come into contact with the skin are safe and suitable for use.